Big money to be saved by using accurate property models in CCS
In order to fulfill the objectives of the Paris Agreement, relevant international bodies predict that massive amounts of CO2 have to be captured and permanently stored or utilized (CCUS) [1, 2]. Finding the best solutions in terms of costs and safety is key to achieve large-scale CCUS deployment both in the short and long term. Among other factors, such an optimization will depend on accurate and robust models for the fluids involved. Currently, these models need to be improved, particularly with respect to the impact of impurities [3].
International collaboration in NCCS provides better models as well as scientific careers and mobility opportunities
The Norwegian CCS Research Centre (NCCS) is systematically improving models both for fluid properties and flow phenomena. The NCCS work on fluid property models is a continuation of a decade long collaboration between leading international research groups. Namely NCCS SINTEF Energy Research, Ruhr-Universität Bochum (RUB), Norwegian University of Science and Technology (NTNU), University of Western Australia (UWA) and NIST are engaged.
Equations of state are probably the most important property models providing all thermodynamic properties of pure components or mixtures of components, like their density, phase behaviour, or heat capacity. RUB is developing a new highly accurate equation of state for CCS called EOS-CG 2019 [4, 5]. SINTEF is developing other models, with focus on robustness and stability [6]. NIST is collaborating in the modelling work.
Learn more about CCS
Join our newsletter to stay updated with all the latest research results and news from NCCS: The Norwegian CCS Research Centre.
In order to develop and verify accurate equations of state, accurate data on thermodynamic properties are needed. It has been identified that the data situation is poor for many relevant mixtures for CCS, which is why measuring accurate thermodynamic data has been an important activity of SINTEF Energy Research for several years using the purpose-built CO2Mix phase equilibrium facility.
To move this work forward, exchange of personnel and knowledge building have been essential. Hence, NCCS PhD candidate Tobias Neumann, working on EOS-CG 2019, has been hosted by NTNU for two years before returning to RUB. From NTNU, the, at that time, master student Sindre Ottøy was engaged as summer researcher for a specialization project and his master thesis working on the CO2Mix facility and analyzing its output. The two have been central in the work to be presented below. Also, other scientists have been visiting the CO2Mix facility over the years, though, which is something we certainly also will welcome in the future and which can be facilitated through the European Carbon Dioxide Capture and Storage Laboratory Infrastructure (ECCSEL).
Now, back to these models: They are built on binary mixtures, but what about real process fluids with multiple components?
Most real process fluids have multiple components. However, thermodynamic models for mixtures are normally built on sub-models describing the behavior of pure components and models for two-component or binary mixtures. The idea is that mixtures with more than two components can be described by combining these pure component and binary models in a clever way. The reason for this simplification is a matter of data availability. In order to fit models, there have to be representative data available, as discussed in a previous NCCS blog article. That is unfortunately usually not the case for mixtures with more than two components, as each additional component means that there is one new dimension in the data matrix. The implication is that already for a 3-component, or ternary, mixture, a full cover of accurate measurements is very resource and time consuming and hence normally infeasible.
However, building up fluid property models for multi-component mixtures based on binary mixtures means that possibly more complex phenomena caused by mixing three or more components together are not taken into account. Hence, it is important to verify fluid property models for multicomponent mixtures through multicomponent measurements, in particular at technological relevant conditions.
NCCS has verified equations of state for a central ternary mixture
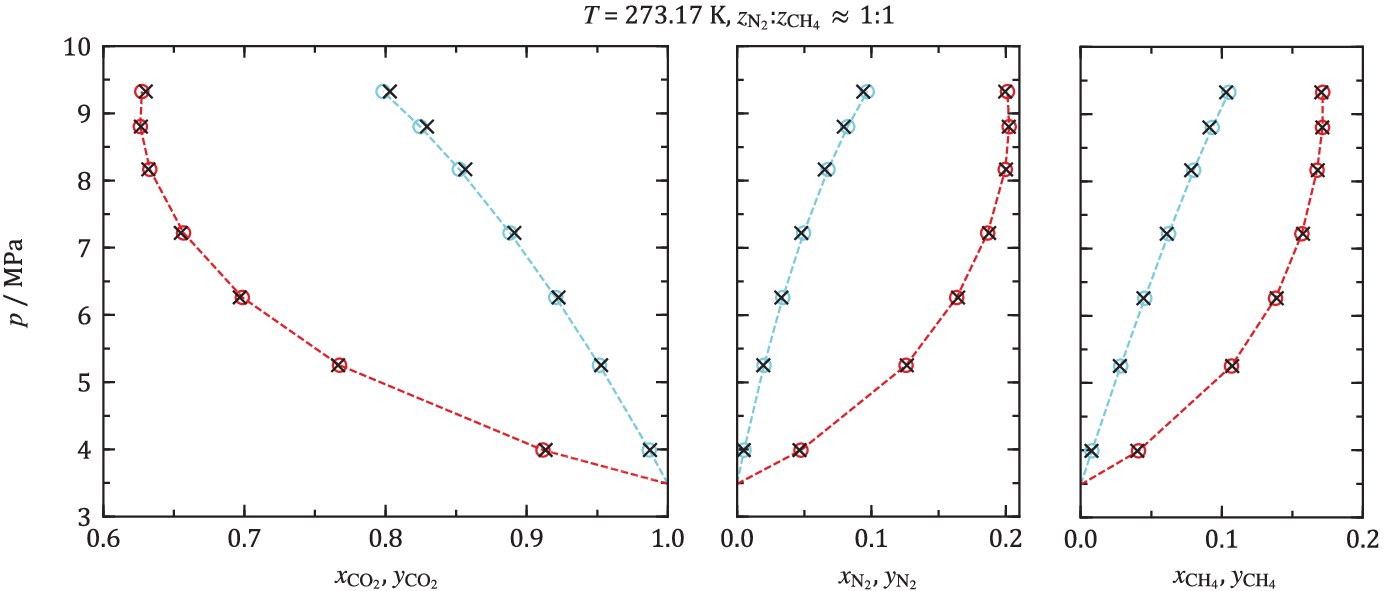
Such a verification was the purpose of an article recently published from NCCS in an open access article in Fluid Phase Equilibria [7]. Phase equilibria, i.e. bubble and dew points, of ternary mixtures of CO2 and the technological important impurities methane and nitrogen were measured. Measurements were performed between -60 and 25 °C, and measurements at 0 °C are shown in figure below. For ternary mixtures, the phase equilibria function becomes a three-dimensional body at a given temperature. As discussed above, the data illustrated is therefore not sufficient to build models. The experimental data were instead compared with the output of EOS-CG 2019. To cut a long story short, the conclusion of this study was that for this ternary system, and at the investigated conditions, a model built on binary mixture data works quite well.
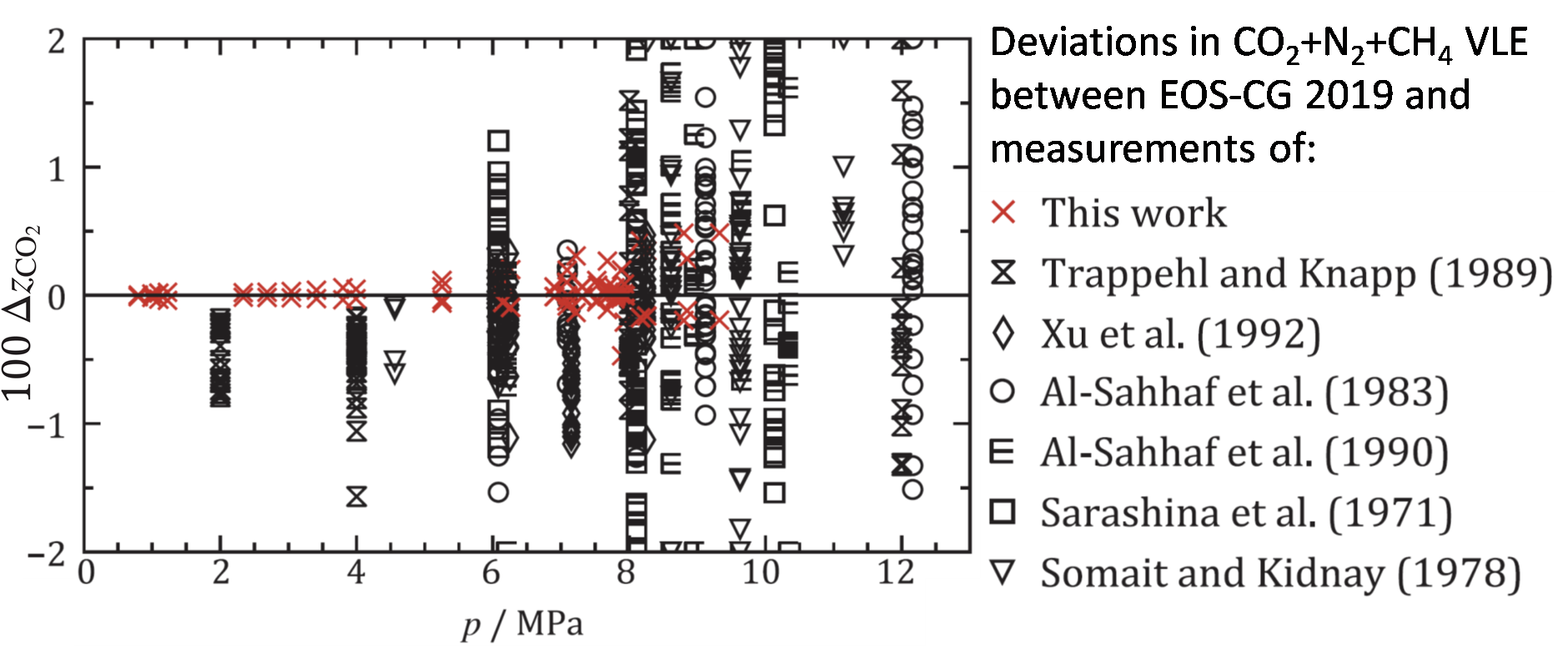
This is illustrated in Figure 4, where the deviations between our model and our new data are shown in red. There are some minor deviations at higher pressures. But these are in fact similar to deviations found between EOS-CG 2019 and binary data of the same components [8, 9], and cannot be attributed to the fact that we were looking at the two impurities together in the new measurements. As can be seen in the figure, there are also previous literature data for the same ternary system. However, apart from being measured mostly at different conditions, they were generally of apparent lower quality and therefor unfit for a confident model verification of this system. Hence, high-accuracy measurements, as provided by the purpose built CO2Mix phase equilibrium facility, are really important to build trust for property models.
Although the EOS-CG 2019 for this ternary system and the new data are in a good agreement, that does not mean that the model performs as well for other CO2 -rich multi-component mixtures. Further, the output of other models might differ. Hence, it is really necessary to continue investigating other multi-component mixtures as well as closing remaining knowledge gaps for binary mixtures.
Learn more about CCS
Join our newsletter to stay updated with all the latest research results and news from NCCS: The Norwegian CCS Research Centre.
References
- World Energy Outlook 2019, International Energy Agency, Paris, France, 2019.
- Special Report on Global Warming of 1.5 °C, Summary for Policymakers. , IPCC 2018.
- Munkejord ST et al. Appl. Energy 2016;169:499-523.
- Gernert J, Span R. J. Chem. Thermodyn. 2016;93:274–93.
- Herrig S, “New Helmholtz-Energy Equations of State for Pure Fluids and CCS-Relevant Mixtures,” Doktor-Ingenieur Thesis, Fakultät für Maschinenbau Ruhr-Universität Bochum, 2018.
- Wilhelmsen Ø et al. Industrial & Engineering Chemistry Research 2017;56:3503-15.
- Ottøy S et al. Fluid Phase Equilib. 2020;509:112444.
- Westman SF et al. Fluid Phase Equilib. 2016;409:207-41.
- Petropoulou E et al. Fluid Phase Equilib. 2018;462:44-58.









Comments
No comments yet. Be the first to comment!