In NCCS (NCCS Task 7) we work to improve and expand our knowledge on the design and operation of safe and efficient CO2-transport systems. Part of this is to understand CO2 flows that are out of equilibrium.
I’ll try to give a flavour of what we mean by non-equilibrium here: Two quantities in non-equilibrium, if left alone for a while, will tend to approach each other. Also, two quantities in equilibrium can get into non-equilibrium if conditions change.
Bike example
Many of you have experienced an example of the latter while happily riding your bike on an asphalt road, and suddenly finding yourself in a curve with gravel on top of the asphalt. The gravel changes the maximum transversal force the bike wheels can transmit to the ground, so that the bike starts moving sideways. The new equilibrium state is you and the bike lying on the ground – hopefully without too many scratches.
Models is a more or less simplified wiew of a part of the physical world
In Task 7 we are not concerned with bikes, but with slightly analogous dynamic situations which we need to model. A model in this context is a more or less simplified view of a part of the physical world, along with mathematical expressions describing this view. We need experiments to assign values to coefficients appearing in the equations. Conversely, the equations help us set up the relevant experiments.
Ship transport of CO2
Ships are seen to be an efficient way of transporting CO2, especially for long distances or when flexibility is needed. One design question is at what pressure (and temperature) the CO2 should be in while in transit.
A relatively low pressure of about 6–7 bar is generally seen as efficient, but this is close to the state where solid CO2 (dry ice) is formed. One does not want solid CO2 to block e.g. valves and pipes. Here the non-equilibrium comes in. We need to know under which conditions solid CO2 is formed, and how much. Once it’s there, we need to know under which conditions the solid CO2 sublimes (turns into gas) or melts, and how fast.
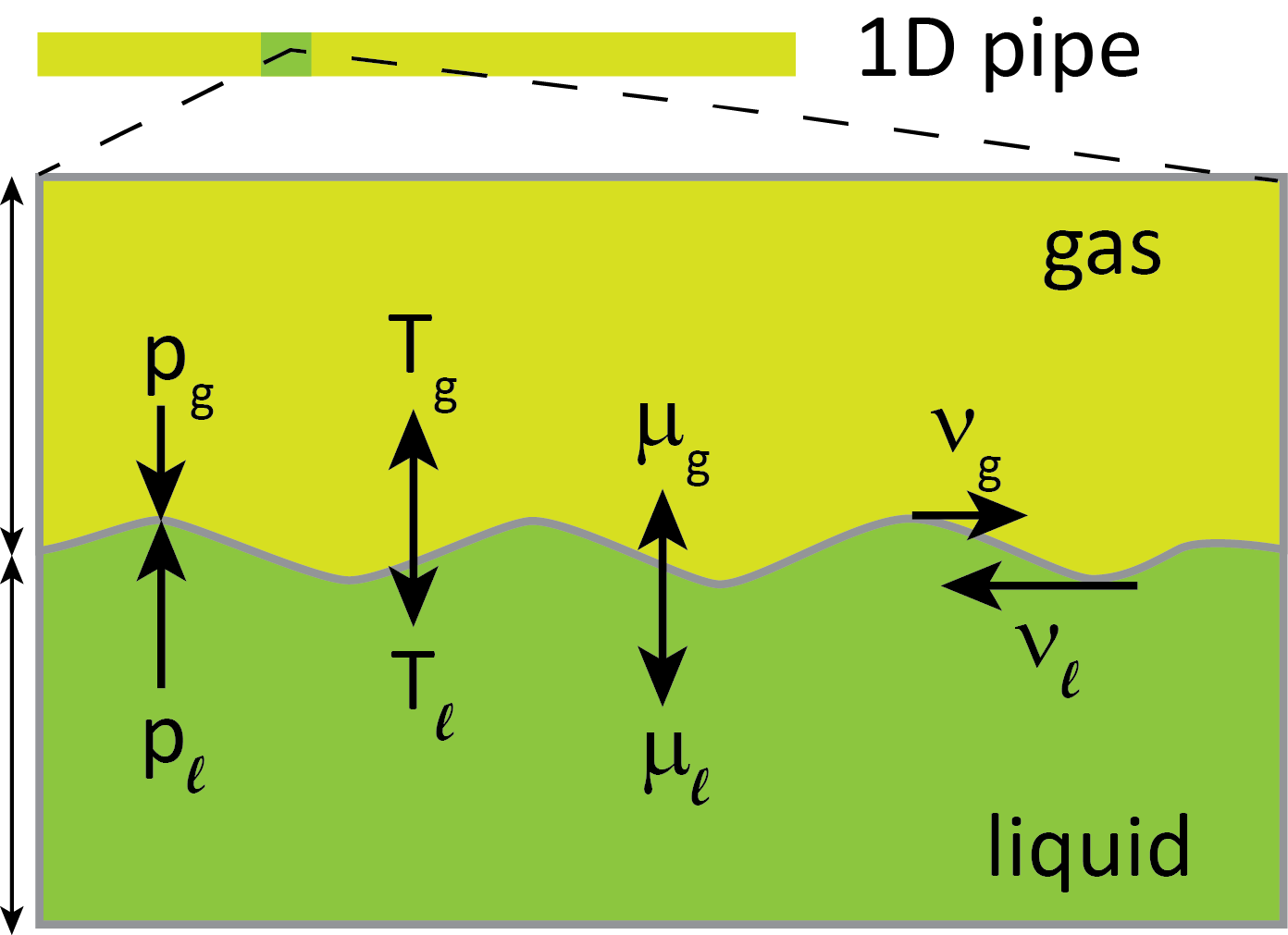
Pipeline transport of CO2
If somehow a hole is formed in a CO2-transport pipeline, it is important that it does not develop into a long running-ductile fracture. See our blog (Safe CO2 transport pipes is part of the climate solution) for more information on this subject.
In order to design pipelines, we would like to be able to describe the fast physics involved during a running-ductile fracture. It all happens within some tens of milliseconds. This may not be long enough that the fluid can be thought to be in equilibrium. Here we need to consider the liquid and the gas phase that both will be present during a depressurization.
The two phases can be out of equilibrium in several ways. They may have different pressures, temperatures, chemical potentials and velocities, see the above figure. We have the hypothesis that if we account for these phenomena, then we will also be able to describe the running-ductile fracture more accurately, which in turn will enable us to design and operate pipelines with greater confidence, and also to evaluate the use of existing hydrocarbon pipelines for CO2.
PhD project
As one may imagine, bringing non-equilibrium into the fluid flow models increases the complexity. This is the price to pay for better accuracy and robustness. Task 7 will study several aspects related to non-equilibrium flow of CO2. In particular, one PhD will be educated on the subject, in a collaboration between NTNU and SINTEF. The PhD candidate, yet to be recruited, will enlighten the subject both from the modelling and the experimental side. Depressurization experiments will be performed in the ECCSEL depressurization facility, which is currently under construction at the roof of the NTNU-SINTEF Thermal Engineering Laboratories.
In this work, we will span the gap from experimental observation and mathematical description of physical phenomena, to industrial applications. This is a way of reducing CCS-project risks and therefore accelerating CCS deployment.











Comments
No comments yet. Be the first to comment!