With new electrolysis technology, industrial waste streams could become a “goldmine” for the critical raw material manganese.
Manganese (Mn) is a vital material for the green transition and an essential component in many technologies and products. Manganese oxide appears in lithium-ion battery cathodes alongside nickel and cobalt oxides. Metallic manganese is primarily used in steel production and also in alloys with other metals such as aluminium. The raw material for producing metallic manganese is either ore, which is mined, or secondary sources like recycled materials and industrial waste streams.
Utilizing secondary sources helps reduce environmental impact, enhances circularity, and lowers supply risk. And the potential is significant: Between 2007 and 2010, more than 20,000 tons of sludge containing manganese was deposited each year from the manganese alloys industry in Norway. This sludge is a byproduct of furnace gas scrubbing and consists of water, dust particles, and other off-gas components – typically containing 20–30% valuable manganese.

Electrolysis technology produces pure manganese products
Most metallic manganese is produced in alloys with iron or silicon in smelting processes and is further used for steelmaking. To obtain pure manganese metal – free of alloying elements – electrolysis is necessary. This product is known as electrolytic manganese metal (EMM), and is used in aluminium alloys, electronics, and chemicals. Today, China produces more than 95% of the global supply (source: RMIS – Raw Materials Information System, European Commission).
Electrolysis can also be used to produce electrolytic manganese dioxide (EMD), a material widely used in alkaline batteries – the kind you find in remote controls, radios, calculators, watches, cameras, and more.
“Two birds with one stone”
Currently, EMM and EMD are produced in separate processes. Through a SINTEF-supported project, researchers have merged the two separate production methods into a single electrolysis process. This enables simultaneous production of two valuable products at nearly the same energy cost. Specifically, producing 1 kg of manganese metal also yields 2.5 kg of manganese dioxide as a co-product – without increasing the energy consumption (7–8 kWh in total).
“Producing two separate products from the same raw material in a single process reduces costs, energy consumption, and the amount of waste requiring special treatment or disposal,” says researcher Siri Marie Skaftun.
In short, valuable sludge from industrial waste streams can be utilized in a cost-effective and sustainable way.
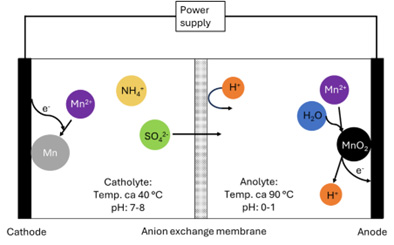
Manganese at the cathode, manganese dioxide at the anode
In the combined electrolysis process, manganese dioxide forms at the anode while manganese metal forms at the cathode. To ensure high product quality and low energy consumption, two different electrolytes are used: one for producing manganese metal (catholyte) and one for producing manganese dioxide (anolyte). A membrane separates the catholyte and anolyte.
Different electrolytic conditions required
Manganese metal requires a low electrode potential to form, so during its deposition at the cathode, some hydrogen gas is inevitably produced as a side reaction. This hydrogen evolution ‘steals’ energy from the process and increases the energy consumption. To minimize hydrogen production, the electrolyte composition must be optimized. The catholyte should have a high pH (7–8), but not so high that manganese ions precipitate as manganese hydroxide, since this would reduce the amount of available raw material. Over time, hydrogen evolution increases the catholyte pH, so a buffer is added to stabilize it. Temperature also plays a key role in energy use. To get a smooth metal surface and avoid short circuits in the cell, the process is usually run at around 40°C.
When producing high-quality manganese dioxide, the electrolyte needs to be much more acidic than when making manganese metal – with a pH around 0–1. The temperature is also different: the best results are achieved with temperatures around 90°C.
To maintain these two different temperatures in one process, the project team separated the anolyte and catholyte into individual containers with controlled temperatures. The anolyte and catholyte were continuously circulated between the containers and the electrochemical cell where deposition of manganese metal and manganese dioxide occured.
Toward a greener, more circular industry
By leveraging new electrolysis technology and tapping into the large amounts of manganese found in industrial waste streams, waste can be turned into valuable products – helping create a greener and more circular industry. The new process also saves energy, benefiting both society and the environment.














Comments
No comments yet. Be the first to comment!