Comparing solvents from different CO2 capture plants allows us to see what can make them last longer and capture more CO2. We have found that the solvents degrade quite differently, depending on their composition, but more research on what is bad and good for the solvent stability is still needed.
One of the easiest ways of capturing CO2 is by bubbling the gas that contains it through a liquid that binds CO2 but lets other gases like nitrogen and oxygen pass through. Really good CO2 capture solvents often also react with other things, like oxygen, to form new compounds, that are bad for the process and that aren’t good at capturing CO2.
The solvents can react with other things than just CO2
CO2 capture solvents have to endure much more than just capturing CO2. In the cyclic process of CO2 absorption and temperature-induced desorption, it will be exposed to temperature stress and encounter other reactive components of the flue gas its cleaning, such as oxygen, SOx and NOx gases, as well as contact with the construction material, which is typically stainless steel. All these variables alter the composition of the solvent over time, where both organic degradation compounds, formed when the solvent reacts with other things than CO2, and inorganic components from the construction materials, or flue gas accumulate in it.
Degradation causes problems in the process
This phenomenon can lead to operational challenges, as well as increase the occurrence of degradation reactions in the solvent by catalysis, and corrosion of the equipment. To combat this, the solvent needs to be cleaned, or “reclaimed” periodically, to remove harmful contaminants. To this point, it has been assumed that the less contaminants you have in the solvent, the less problems you will have. In particular, it has been assumed that solvent degradation can be kept at bay by removing all contaminants in the solvent. Is this really true though? We don’t think so!
Solutions from different CO2 capture plants degrade at different speed
In our most recent scientific publication, we took a common solvent, ethanolamine, from three different sources, that had been used for CO2 capture from three different industrial flue gases and tested these in the labs at NTNU. The lab experiments showed that oxidative degradation, the dominant degradation mechanism in the CO2 capture plants, varied a lot in the three different solutions. But is the “dirtiest” solvent the least stable?
Some used solutions work better than the fresh ones
While one of the solutions degraded faster, two of them were actually more stable than an ethanolamine solvent that had not been in contact with real flue gas or the pilot plants! With analytical methods at SINTEF, we characterised the all the solvents before and after our lab tests, meaning that we found out exactly how much of each degradation product and contaminant was in there. Here we found that not only did their composition of organic and inorganic contaminants vary due to the different plant designs and flue gases, but they also seemed to follow different degradation mechanisms in the process. This could mean that not all contaminants need to be bad for the solvent.
Some things reduce, others increase solvent degradation
Based on the results, one could be tempted to conclude that some inorganic contaminants may reduce degradation, while others increase it. However, as the solvent mixture in a plant gets very complex with various contaminants over time, it is too early to draw final conclusions.
More research is still necessary
More work is needed to what exactly is bad and what is good for the solvent’s stability. For example, what is the role of organic contaminants? Do some of them actually increase, or reduce the reactivity of the inorganic compounds, such as metals? This is something we want to look at in our future work. By understanding these phenomena better, we can save waste and energy, by not performing unnecessary cleaning, or reclaiming of the solvent, and keep it in operation for longer!
This work was performed as a part of the Norwegian CCS Research Centre: NCCS.
Link to the article: https://doi.org/10.1016/j.ccst.2023.100110
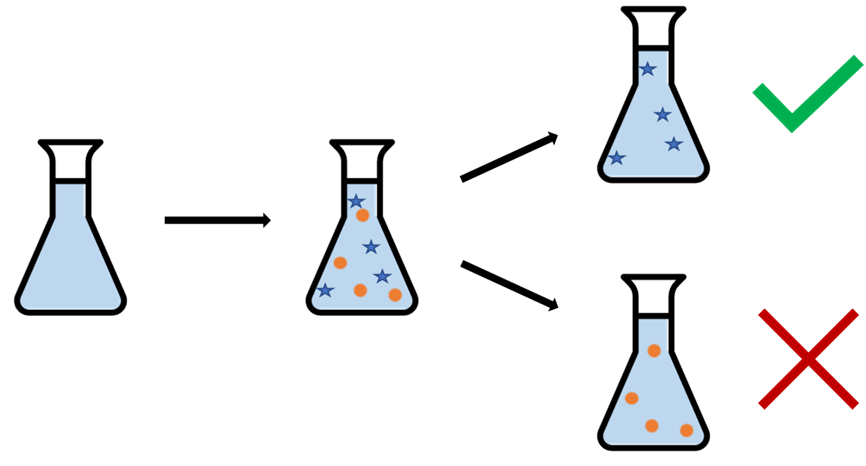
Figure 1: During the CO2 capture process, our CO2 capture solvent ends up filled with things that weren’t there to start with. We see that some of these contaminants that show up in the process are actually beneficial, and increase the lifetime of the solvent, while others can be bad and reduce it.












Comments
No comments yet. Be the first to comment!