In the transition towards more sustainable energy systems, energy storage has a big role to play. Heat batteries, or thermal energy storage (TES), have been gaining more and more attention as the missing link between heat production and heat demand, and as a way to make use of otherwise wasted heat. However, today’s TES systems are hampered by low energy density, and may thus be large and voluminous. One of the new routes currently explored is thermochemical energy storage (TCES), which can offer more compact heat storage, with the added benefit of not losing much of the stored heat, paving the way for long-term energy storage.
Why thermal batteries?
On top of the already imminent climate crisis, the current energy situation brought on by the Ukrainian war has spurred the need for a rapid change of European energy systems. In Norway, high electricity prices have led to increased focus on energy-saving and energy-shifting technologies. According to the main guidelines proposed by the International Energy Agency, a secure and environmentally-friendly exit from the current energy crisis requires clean energy technologies to be upscaled and fossil fuels to be scaled back. However, most renewable energy systems are volatile – we cannot make the sun shine or the wind blow – which means developments and improvements of energy storage technologies are key to ensure sufficient flexibility and avoid fossil back-up on days of with no sun nor wind.
In short, we need efficient, versatile and non-polluting batteries. The optimal battery depends on the kind of energy being produced and needed. In many cases, and particularly where heat (or cold) is produced and later needed, thermal batteries are the obvious choice. They prove attractive due to their reversibility and integration possibilities in systems with varying demands, such as solar and wind farms, and can be used to recover excess heat from industry. With the right choice of materials, thermal batteries are safe, inexpensive and have a low environmental impact. They are commonly referred to as thermal energy storage.
Thermal energy storage (TES) materials can store heat or cold through their physical/chemical properties and release it hours, days or even months later. Depending on the behavior of the TES material with temperature variations, there are 3 main systems of TES (see Figure 1):
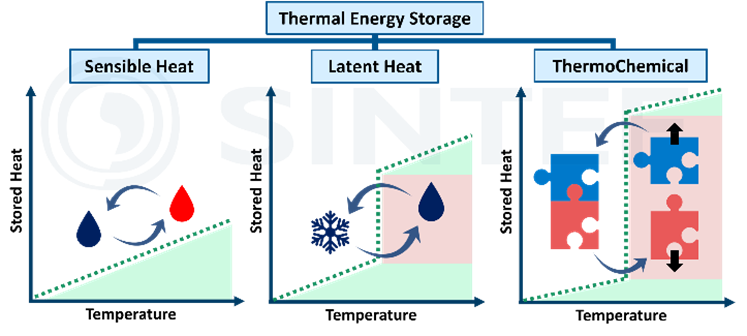
- Sensible TES is heat storage through traditional heating of a material, such as hot water tanks or heated soapstone. In a sensible TES material, temperature and energy are proportional, so the more energy you put into the material, the higher its temperature will be.
- Latent TES takes advantage of the heat absorbed or released in a phase transition of the material, usually between solid and liquid phases. The typical example is ice used to keep food or drinks at zero degrees – the phase change temperature – until everything has melted. The energy stored in these transistions is considerably higher than sensible heat. For water, approximately 80 times more energy is present in melting the material, than in raising the temperature from 1 to 2 degrees. Materials employed in latent TES technologies are called Phase Change Materials (PCMs). More info in this previous blog article What are Phase Change Materials? and in the report Phase change materials for thermal energy storage in low‐ and high‐temperature applications: a state‐of‐the‐art.
- Thermochemical energy storage (TCES) materials store heat through reversible chemical reactions. Upon combination or separation of two substances, heat is absorbed or released. TCES materials can generally store more energy than sensible and latent heat TES compounds.
At SINTEF Energy Research, we work on a multitude of TES technologies. A new initiative through energy efficiency research centre FME HighEFF will bring the attention to TCES materials which have yet to reach commercialisation but offer a range of advantages over today’s energy storage materials.
How can TCES be the next generation of thermal batteries?
One of the most interesting TCES systems is water sorption, where one of the reactants in the thermochemical reactions is water, in liquid or steam form.
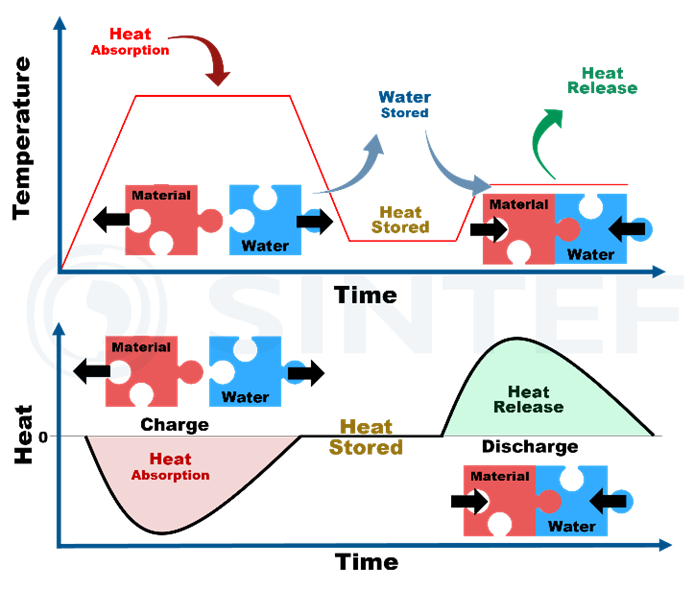
An example of TCES used for heat storage is given in Figure 2. Charging occurs when excess heat is used to increase the temperature, thereby dehydrating the material and releasing water. The material will stay in this “charged” state as long as it is kept in dry conditions. The discharge occurs when the material is hydrated through the introduction of water or steam, which releases the stored energy, generally at lower temperature than the dehydration reaction.
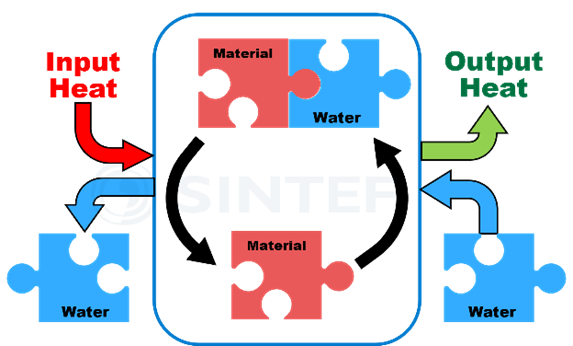
The charge and discharge of a TCES system occur in a reactor, of which there are two main types. In integrated reactors (see Figure 3), water circulates in and out of the reaction media within a closed tank. In this case, the amount of energy stored depends directly on the amount of TCES material.
For separated reactors (see Figure 4), the hydrated material circulates from a low-energy tank through a reactor, where dehydration takes place, to a high-energy tank, where the charged material is stored. Upon discharge, the material is brought through the same or a separate reactor back to the low-energy tank.
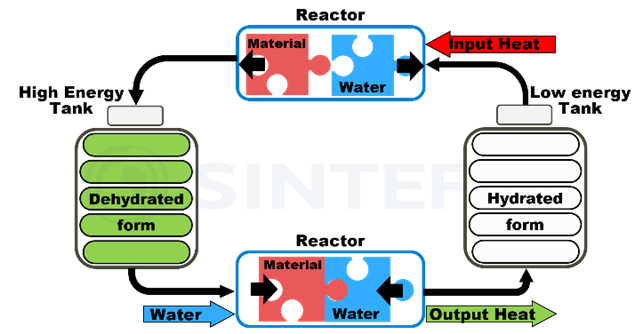
The potential of TCES as a fundamental piece in the transition to clean energy systems relies on its high energy storage capacity and compactness compared to sensible and latent heat TES, where upscaling involves increasing the total system size, with potentially high costs. In TCES systems, this upscaling limitation is overcome by flowing the reactants with separate reactors and storage tanks. Also, as different chemical reactions have different charging/discharging temperatures, TCES materials can be tailored to a large range of temperatures and thereby usages.
At SINTEF Energy Research, we are working on water sorption TCES through the project ITChES (Integration of ThermoChemical Energy Storage), together with SINTEF Industry, within the framework of FME HighEff. In this project, the performance potential of selected TCES compounds, for excess heat recovery in the range of 120-300 °C, is analysed at lab-scale. The results will be used as the base for a techno-economic study, with the aim of paving the way to large-scale implementations in industrial processes.
Thermochemical energy storage is one of the key tehnologies in the green transition, and it is currently in development to become the next generation of thermal batteries that can contribute to a secure and flexible exit from fossil fuels and an efficient transition towards clean energy systems.











Comments
No comments yet. Be the first to comment!