Is hydrogen the fuel of the future?
A car or a power plant that runs on hydrogen only emits heat and water. Norway generates a lot of hydro- and wind-power. Some of this renewable energy can be used to produce hydrogen from water splitting through electrolysis. In addition, part of Norway’s natural gas resources can be converted to clean hydrogen with the use of reforming combined with capture and permanent storage of CO2.
Prize for Young and Outstanding Researchers to Øivind Wilhelmsen from SINTEF
Hydrogen can, together with electricity from renewables, contribute to makeing our lives more environmentally friendly. For instance, Japan is now building cars, production facilities and infrastructure to prepare for the hydrogen society, and the oil and gas industry are watching closely.
Liquid hydrogen or gas-phase hydrogen
Hydrogen is a light gas that takes up a lot of volume. Even when compressed to extreme pressures such as 200 bar, the hydrogen still takes up a lot of volume per unit of available energy. This poses a challenge when exporting large quantities of hydrogen across longer distances, such as from Norway to Japan. A solution to this challenge is to cool down the hydrogen to about -252oC and liquefy it. It is then possible to ship a batch of hydrogen in one ship (liquefied hydrogen) at low pressure, instead of five ships at high pressure (200 bar).

So far, liquid hydrogen has not been required in large quantities for any purpose. Consequently, there has been little technology development on this area. Current state-of-the-art liquefaction plants are inefficient. Nearly 11–12 kWh of energy is needed per kg of hydrogen to be liquefied. This is roughly 25% of the energy that can be exploited in a fuel-cell afterwards, hence a good portion of the energy will be lost before the hydrogen even reaches the customer.
Energy efficient liquefaqtion
At SINTEF Energy Research and the Norwegian University of Science and Technology (NTNU), we want to do something about this! By improving the technology, we believe that it is possible to push the energy requirement to liquefy hydrogen below 6 kWh per kg. Such an accomplishment would make it much more attractive to transport hydrogen efficiently across long distances.
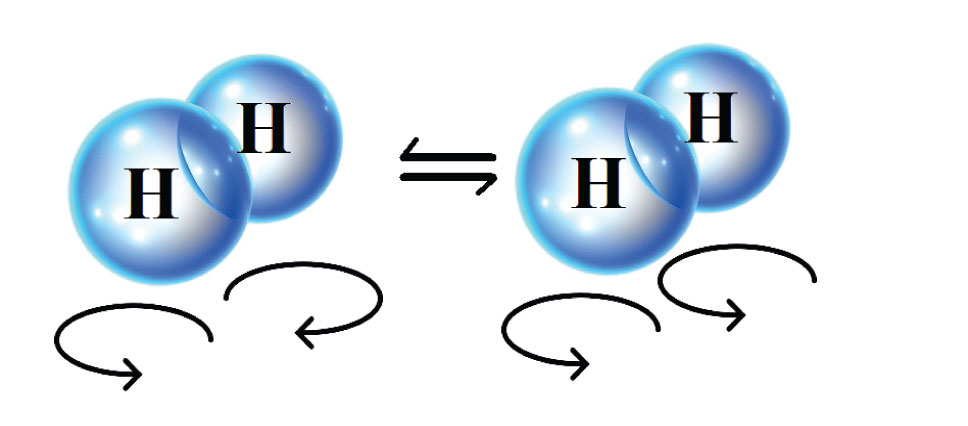
There is a wealth of interesting scientific and technological challenges that need to be overcome before energy efficient hydrogen liquefaction can be realized. For instance, hydrogen consists of two types, called spin-isomers. For one of them, para-hydrogen, the two protons of the hydrogen molecule spin in opposite directions. For the other spin-isomer, ortho-hydrogen, the two protons spin in the same direction. Para-hydrogen is the spin-isomer with the lowest energy and the difference between their energies increases as the temperature becomes lower. In fact, there is so much energy that is released when ortho-hydrogen converts into para-hydrogen, that it is enough to evaporate the hydrogen into its gaseous state again. To prevent this from happening in the storage tanks for liquid hydrogen, one has to speed to up the conversion from ortho- to para-hydrogen by incorporating catalyst pellets inside of the heat exchangers used in the liquefaction process.
Two ways to increase efficiency
In a recent article, we show that there are at least two avenues that can be exploited to increase the efficiency in the cryogenic part of the hydrogen liquefaction process:
- To improve the efficiency of the catalyst pellets
- To use novel refrigerant-mixtures of helium-neon-hydrogen
An important advantage of mixtures of helium-neon-hydrogen is that their composition can be tailor-made so that they evaporate on the cold-side of the heat exchangers. Evaporation can enhance the heat transfer and the efficiency considerably, in particular in the hydrogen liquefaction process. In fact, in the article we show that it is possible to reduce the loss of useful work (the exergy destruction) in the heat exchangers by more than 43% by exploiting both of the avenues described above.
SINTEF and NTNU researchers on research exchange
To achieve this in a safe and precise manner, it is necessary to properly characterize and understand the behavior and the thermo-physical properties of mixtures of helium- neon-hydrogen. For example, at which temperatures, pressures and compositions will such mixtures evaporate? Today, there exist no models that can describe these mixtures to a sufficient accuracy.

Researchers from SINTEF and NTNU have this spring been at a research exchange at Imperial College London to develop models that can be used to accurately describe novel refrigerants for use in the hydrogen liquefaction process. Here, we have been part of the research group of Prof. Erich Müller, a world-leading group on thermodynamics and development of fundamental equations of state. In the few months that we have been here, we have made significant progress on how to understand these fluids under the excellent supervision and guidance of Prof. Müller. Our aim is that by next year, we will have developed unique thermodynamic tools that will help us to improve the efficiency of the hydrogen liquefaction process and take one step towards realizing Norway’s perhaps next big export article – liquid hydrogen.

The research exchange in London would not have been possible without the joint support from the ENERSENSE strategic research area at NTNU, the research project Hyper and the internally financed HYVA project at SINTEF Energy research. We are very grateful for this opportunity.
Best wishes from London










Comments
Hydrogen could very well be the fuel of the future. But, it is an extremely flammable gas, which poses a hindrance to its transportation. However, if the research by SINTEF and NTNU is fruitful, we may have an answer to this problem too.
Dear Øivind,
It is an interesting article. We mentioned it in an article on our BusinessPortal Norwegen.
http://businessportal-norwegen.com.routing.wpmanagedhost.com/2018/04/26/hannover-messe-norwegen-in-einer-stunde/
Best regards
Jutta Falkner
jutta.falkner@businessportal-norwegen.com
Phone +4930 24 98 665
Mobil + 49 172 322 45 59